Press Release
Akebia Presents Results from its INNO2VATE Global Phase 3 Program; Demonstrated Efficacy and Cardiovascular Safety of Vadadustat for the Treatment of Anemia due to Chronic Kidney Disease in Adult Patients on Dialysis
- INNO2VATE data presented at American Society of Nephrology Kidney Week 2020 Reimagined
- Presentation expands on previously announced positive top-line data showing vadadustat achieved clear and consistent results across primary and secondary efficacy and safety endpoints
- Company on track to submit New Drug Application
- Company to host investor briefing webcast at 4:10 p.m. ET on October 23, 2020
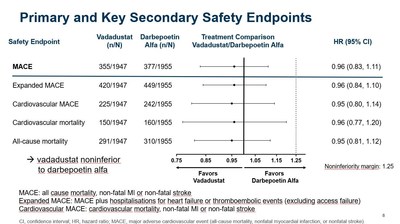
"The data presented today build on the positive top-line efficacy and safety results from INNO2VATE that were previously reported in May. More specifically, vadadustat's cardiovascular safety profile in dialysis patients is further reinforced by newly presented data clearly showing vadadustat achieved non-inferiority to darbepoetin alfa on MACE, expanded MACE, cardiovascular MACE, cardiovascular mortality, and all-cause mortality. These results were also consistent across multiple pre-specified populations," said
Results from INNO2VATE are being presented today at ASN Kidney Week during a presentation titled, "Global Phase 3 Clinical Trials of Vadadustat vs Darbepoetin Alfa for Treatment of Anemia in Patients with Dialysis-Dependent Chronic Kidney Disease" (Abstract TH-OR01).
Highlights of the INNO2VATE ASN Kidney Week Presentation:
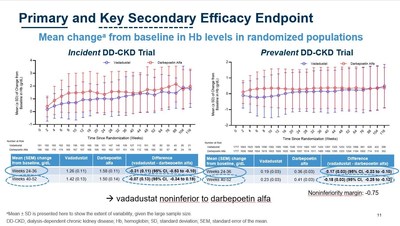
Efficacy:
Safety:
- As previously reported, vadadustat achieved the primary safety endpoint of the INNO2VATE program, defined as non-inferiority of vadadustat versus darbepoetin alfa in time to first occurrence of a major adverse cardiovascular event (MACE), which is the composite of all-cause mortality, non-fatal myocardial infarction (MI), or non-fatal stroke across both INNO2VATE studies.
- INNO2VATE results on key secondary safety endpoints were clear and consistent. Vadadustat demonstrated non-inferiority to darbepoetin alfa in analyses of expanded MACE, cardiovascular MACE, cardiovascular mortality, and all-cause mortality.
- The incidence of treatment emergent adverse events during the incident dialysis patient (Correction/Conversion) study in vadadustat treated patients was 83.8% and 85.5% in darbepoetin alfa treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa treated patients were hypertension (16.2%/ 12.9%) and diarrhea (10.1%/ 9.7%). Serious treatment emergent adverse events were lower in vadadustat treated patients at 49.7% compared to 56.5% for darbepoetin alfa treated patients. The incidence of treatment emergent adverse events during the prevalent dialysis patient (Conversion) study in the vadadustat treated patients was 88.3%, and 89.3% in darbepoetin alfa treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa treated patients were diarrhea (13.0%/ 10.1%), pneumonia (11.0%/ 9.7%), hypertension (10.6%/ 13.8%), and hyperkalemia (9.0%/ 10.8%). Serious treatment emergent adverse events were slightly lower for vadadustat treated patients at 55.0% and 58.3% for darbepoetin alfa-treated patients.
"We could not be more excited and pleased with such compelling results across our clinical development program in patients on dialysis for vadadustat," said
Akebia's vadadustat development program also includes PRO2TECT, the global Phase 3 program for the treatment of anemia due to CKD in adult patients not on dialysis. Results from this program will be presented at ASN Kidney Week in a late-breaking presentation on
Investor Briefing Webcast
Akebia management will host an investor briefing webcast with Dr.
About the INNO2VATE Global Phase 3 Program of Vadadustat
Akebia's global INNO2VATE program included two separate Phase 3 studies for incident dialysis patients (Correction/Conversion) and prevalent dialysis patients (Conversion), which collectively enrolled 3,923 adult patients on dialysis with anemia due to CKD. The INNO2VATE incident dialysis patient study evaluated 369 incident dialysis patients and the prevalent dialysis study evaluated 3,554 prevalent dialysis patients. Both INNO2VATE studies were global, multicenter, open-label, sponsor-blind, active-controlled (darbepoetin alfa - an injectable erythropoiesis stimulating agent), non-inferiority studies. In both studies, patients were randomized 1:1 to receive either oral vadadustat or injectable darbepoetin alfa. Efficacy and safety results were measured against non-inferiority margins agreed upon with the FDA and the EMA.
About Anemia due to Chronic Kidney Disease (CKD)
Anemia is a condition in which a person lacks enough healthy red blood cells to carry adequate oxygen to the body's tissues. It commonly occurs in people with CKD because their kidneys do not produce enough erythropoietin (EPO), a hormone that helps regulate production of red blood cells. Anemia due to CKD can have a profound impact on a person's quality of life as it can cause fatigue, dizziness, shortness of breath and cognitive dysfunction. Left untreated, anemia leads to deterioration in health and is associated with increased morbidity and mortality in people with CKD.
About Vadadustat
Vadadustat is an oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor designed to mimic the physiologic effect of altitude on oxygen availability. At higher altitudes, the body responds to lower oxygen availability with stabilization of hypoxia-inducible factor, which can lead to increased red blood cell production and improved oxygen delivery to tissues. Vadadustat is in global Phase 3 development for the treatment of anemia due to CKD and is not approved by the
About
Forward Looking Statements
Statements in this press release regarding Akebia's strategy, plans, prospects, expectations, beliefs, intentions and goals are forward-looking statements within the meaning of the
Investor Contact
Ir@akebia.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-inno2vate-global-phase-3-program-demonstrated-efficacy-and-cardiovascular-safety-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-on-dialysis-301158444.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-inno2vate-global-phase-3-program-demonstrated-efficacy-and-cardiovascular-safety-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-on-dialysis-301158444.html
SOURCE


Akebia Therapeutics, Inc.
245 First Street, Suite 1400
Cambridge, MA 02142
+1 617.871.2098 phone
+1 617.871.2099 fax